
Notice how the carbons are no longer drawn in and are replaced by the ends and bends of a lines. These molecules correspond to the exact same molecules depicted for Lewis-type structures and condensed formulae. If you work with a molecular model kit you will find it difficult to make stick straight molecules (unless they contain sp triple bonds), whereas zig-zag molecules and bonds are much more feasible. Notice how COOH means C(=O)-O-H instead of CH 3-C-O-O-H because in the latter structure carbon does not have a complete octet and oxygens.īecause of the typical (more stable) bonds that atoms tend to make in molecules, skeletal chains often end up looking like zig-zag lines. In example C, the carbon is double bonded to oxygen and single bonded to another oxygen. As you read through a a condensed formula, if you reach an atom that doesn’t have a complete octet by the time you reach the next hydrogen, then it’s possible that there are double or triple bonds. Also, notice the -OCH 3 is in written in parentheses which tell you that it not part of the main chain of carbons. As you go through a condensed formula, you want to focus on the carbons and other elements that aren’t hydrogen. The hydrogens are important, but are usually there to complete octets.
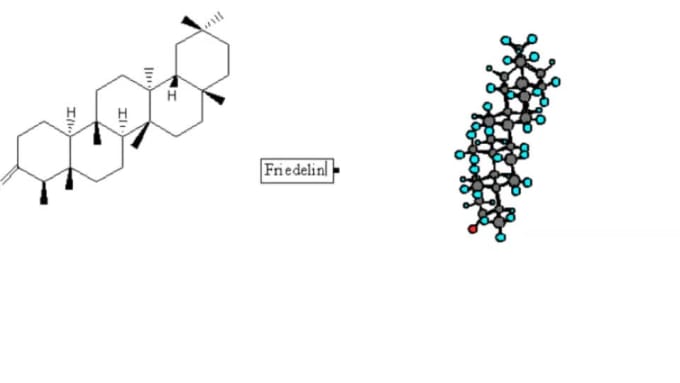
(A) CH 3CH 2OH (B) ClCH 2CH 2CH(OCH 3)CH 3 (C) H 3CNHCH 2COOH Condensed formulas can be read from either direction and H 3C is the same as CH 3, although the latter is more common because Look at the examples below and match them with their identical molecule under Kekulé structures and bond-line formulas. The majority of the drawing uses the skeletal formula, but the -CH 3 are written as condensed formulae, and the -OH group is written in Lewis-type form.Ī condensed formula is made up of the elemental symbols. The order of the atoms suggests the connectivity. It will be more helpful if you become comfortable going from one style of drawing to another, and look at drawings and understanding what they mean, than knowing which kind of drawing is named what.Īn example of a drawing that incorporates all three ways to draw organic molecules would be the following additional drawing of retinol. The different ways to draw organic molecules include Lewis-type, condensed formulae, and skeletal formulae. Through general chemistry, you may have already experienced looking at molecular structure. Another good idea is to get a model kit and physically make the molecules that you have trouble picturing in your head. At first it may seem difficult or overwhelming, but the more you practice looking at and drawing organic molecules, the more familiar you will become with the structures and formulae. Some people say that organic chemistry is like another language, and in some aspects, it is. Learning and practicing the basics of organic chemistry will help you immensely in the long run as you learn new concepts and reactions. In addition, some of these shorthand ways of drawing molecules give us insight into the bond angles, relative positions of atoms in the molecule, and some eliminate the numerous hydrogens that can get in the way of looking at the backbone of the structure. It is tedious to constantly draw out every detail, especially when not necessary, so organic chemists developed ways to make it more convenient and easy. Why were different drawing techniques developed? Organic molecules can get complicated and large.
Structure to name chemdraw how to#
Here you will learn how to understand, write and draw organic molecules. 1: Representations of 1–bromobutane: Skeletal structure, Lewis–type structure, InChI string, InChIKey, SMILES and CAS Registry Number. 1 illustrates some of the most common representations for the compound with the name 1-bromobutane. Sometimes structures need to be represented in other ways, such as a name or other identifier.

Structure to name chemdraw full#
Full Lewis structures are used only rarely, when a more complete drawing is needed, because they are often harder to read than skeletal structures. Chemical structures are usually represented by the skeletal formula, which provides a graphical representation of the molecule with most hydrogens omitted for clarity. They make up the “words” of every organic chemistry sentence, so it is vital to understand how to read and write structures. Representing a chemical structure Chemical structures are the essential building blocks in organic chemistry.


 0 kommentar(er)
0 kommentar(er)
